An analysis into Surface Tension – The missing link to a good prime coat?
I hope the title caught your attention! Below is a summary of the findings presented for the EICF European Casting Conference held in Porto in April of 2018.
As part of the work, we investigated what affects the use surfactants have on the prime slurry surface tension and resultant prime coat adhesion via plate weights.
What is surface tension?
Surface tension is an interesting phenomenon. Surface tension is the tension caused by the attraction of the particles on the surface layer. Many liquids have very different surface tensions based on the internal molecular forces within the liquid as per figure 1.
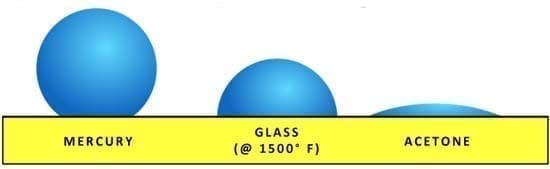 Figure 1 Surface tension of different liquids (source: fusedglass.org)
Figure 1 Surface tension of different liquids (source: fusedglass.org)
Interestingly, it can cause phenomena enabling insects to walk on water and retains water on leaves for increased absorption during heavy rainfall.
 Figure 2 Some insects can walk on water due to surface tension. Water is also retained on leaves due to surface tension between the leaf and water
Figure 2 Some insects can walk on water due to surface tension. Water is also retained on leaves due to surface tension between the leaf and water
Surface tension is important for prime slurries, as you can see within figure 2, the wetting of the material onto the surface changes based off the surface tension of the material. The use of a surfactant acts to reduce this wetting angle and improve the wetting of the material.
 Figure 3 Wetted and unwetted liquids on a glass substrate
Figure 3 Wetted and unwetted liquids on a glass substrate
Secondly, the use of a wetting agent improves the wetting of the refractory within the slurry. The dispersion and coating of the refractory is required before the slurry can stabilize viscosity and flow. Figure 4, shows a quick lab trial with the different wetting behaviour of two identical slurries with and without wetting agent.
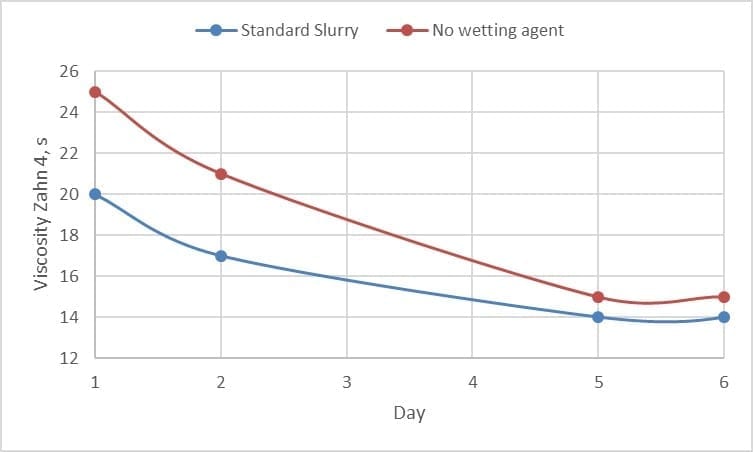 Figure 4 Effect of wetting agent on the wetting in of slurries with and without the presence of a wetting agent
Figure 4 Effect of wetting agent on the wetting in of slurries with and without the presence of a wetting agent
How do you measure surface tension?
There are many ways of measuring surface tension, these depend on whether the material is liquid or solid. Below is a brief synopsis of the most popular methods of measurement:
Capillary Method – The surface tension of liquids can be calculated by measuring the contact angle of liquids in a capillary tube as per Figure 5.
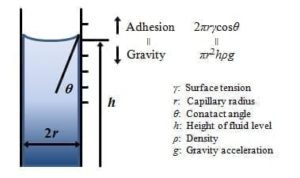 Figure 5 Capillary Surface tension measurement system and equation
Figure 5 Capillary Surface tension measurement system and equation
Sessile drop point – This method uses a drop of liquids of known surface tension and a solid substrate. The resultant contact angle made between the materials can measure the surface energy of the solid. This method can be sued to measure the surface of the liquid or the surface energy of the substrate solid.
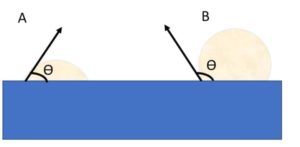 Figure 6 Wetted (A) and unwetted (B) liquids on a wax substrate x is the angle which can be used to calculate the surface tension of both the poolar and dispersive part of the liquid
Figure 6 Wetted (A) and unwetted (B) liquids on a wax substrate x is the angle which can be used to calculate the surface tension of both the poolar and dispersive part of the liquid
Wilhelmy Plate – This method analyses the force required to break the surface tension when the plate is slowly removed from the liquid. The plate is of a known perimeter length and the surface tension can be calculated.
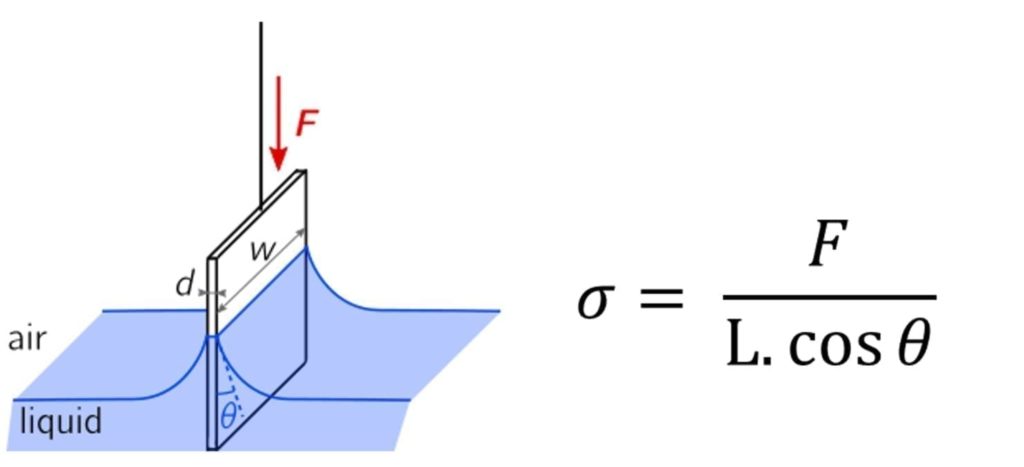 Figure 7 Wilhelmy Plate apparatus and Equation
Figure 7 Wilhelmy Plate apparatus and Equation
Du Nuoy Ring – This method uses a ring instead of a plate as above to measure the force required of a sample. There are subtle differences between this method and the Wilhelmy plate. Namely, there is a non- equilibrium present in the Du Nuoy method vs. the Wilhelmy plate. The du Nuoy method the ring is pulled through the surface, while in the Wilhelmy method, the plate is stationary, and the liquid is static.
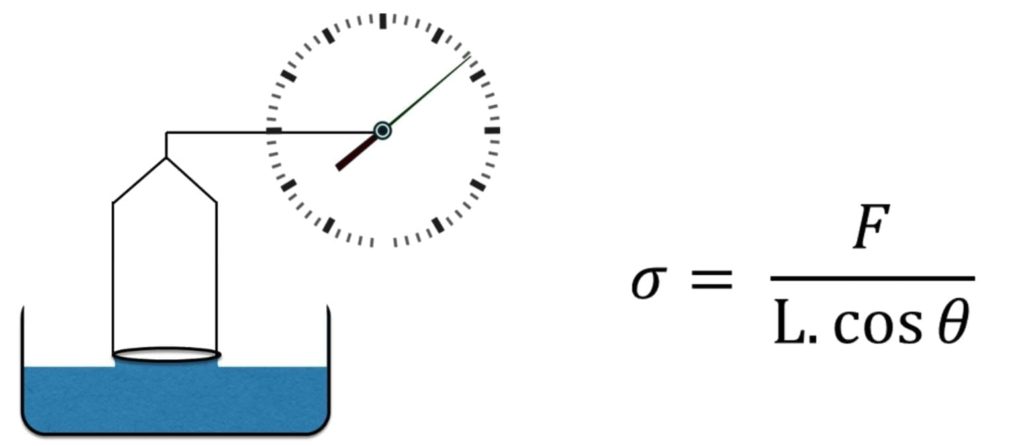 Figure 8 Du Nuoy Ring apparatus and Equation
Figure 8 Du Nuoy Ring apparatus and Equation
Platinum iridium metal is generally used as the ring which has a wetting angle of zero. This makes the surface tension of the sample directly related to the force required to lift the ring form the liquid. This is the preferred method for measuring surface tension of binders. Figure 9 shows the setup Remet UK have within their laboratory.
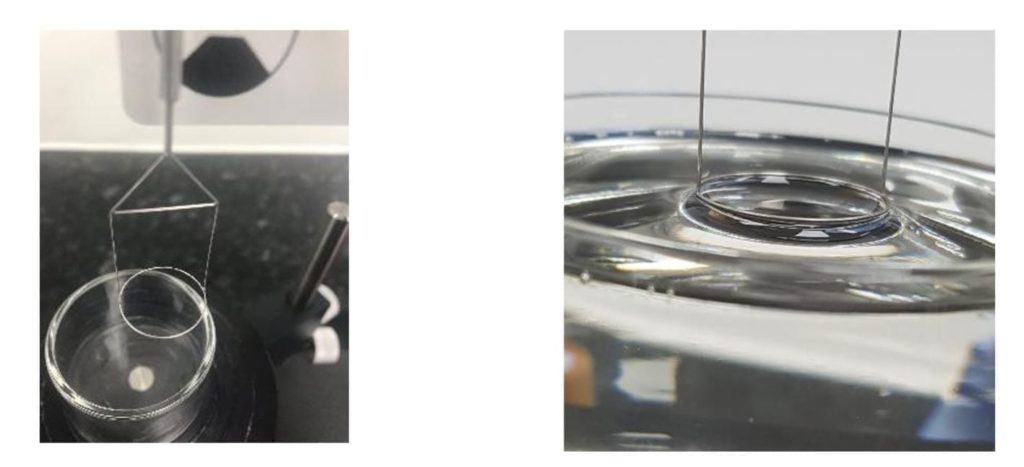 Figure 9 Du Nuoy apparatus within Remet UK
Figure 9 Du Nuoy apparatus within Remet UK
Base binder Surface Tension
The surface tension of many binders is consistent and are independent of silica surface tension or whether the binder is aluminized. The small particle binders have slightly less surface tension, but they are all equivalent once surfactant is added to the binder. This was an interesting finding which shows that the wetting of these binders is independent of the particle size within the colloid in the presence of a wetting agent.
 Figure 10 Effect of Binder properties on surface tension of materials
Figure 10 Effect of Binder properties on surface tension of materials
Effect of surfactant Concentration
The surfactant within the slurry has the function of reducing this surface tension. Analysis was conducted on the concentration levels required to fully wet the slurry out and ensure the wax is fully coated. The results for this testing are outlined below within Figure 11.
 Figure 11 Surface Tension of an alkaline small particle slurry with Varying Concentrations of Surfactant
Figure 11 Surface Tension of an alkaline small particle slurry with Varying Concentrations of Surfactant
Finally, we reviewed if the presence of the refractory, polymer and other additives had on the surface tension of the binder. Samples were mixed together and then the supernatant was tested after a one-week period. There was found to be no degradation of the surface tension over this time. However, it is known that during dipping, the surface tension can change over time and wetting agent may be required to be added periodically to ensure good wetting of wax parts.
 Figure 12 Surface tension of unmixed and mixed slurry after one week
Figure 12 Surface tension of unmixed and mixed slurry after one week
Plate Weight
So, now that we know what surface tension is, how it is measured, and the effect so slurry and surfactants on the value, how does this property effect the process?
To analyses this, two samples of slurry were taken, one fully wetted, the other un‑wetted. These were mixed simultaneously and then measured for plate weight over time. As per the Figure 12 below, the slurry retained after 2 minutes was 11% higher for the wetted slurry! This points at the wetting of the plate and subsequent retention higher for a wetted slurry.
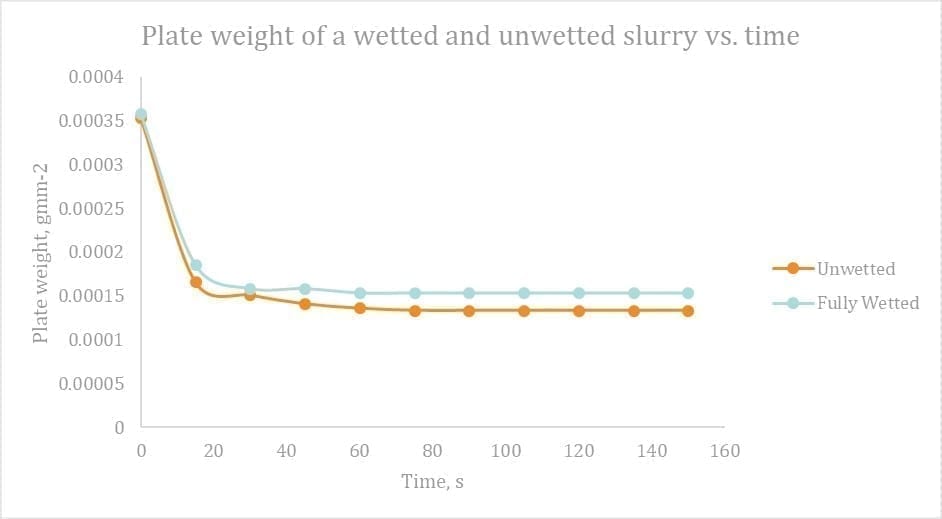 Figure 13 Plate weight of a wetted and unwetted slurry vs. time
Figure 13 Plate weight of a wetted and unwetted slurry vs. time
So, given those results – Should we dump in loads of surfactant into the slurry? The simple answer is no. There is a fine balance between the slurry being wetting and the subsequent bubble formation. As shown below, although surfactant can reduce the surface tension, the knock-on effect is increased bubble formation which is detrimental to a casting.
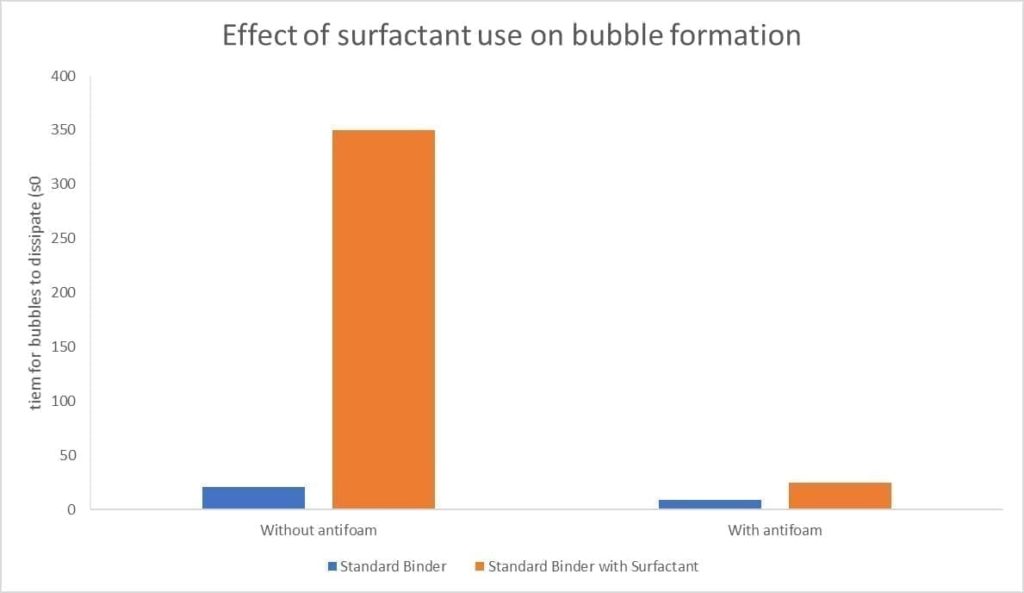 Figure 14 Effect of surfactant use on bubble formation
Figure 14 Effect of surfactant use on bubble formation
Conclusion
This article has introduced the concept of surface tension for slurries and binders, it’s effect on prime slurry characteristics and how it changes within a slurry. Remet UK will be further developing this method and gaining results to increase the knowledge gained through this test for our customers.
< Back to insights



